Oncology Case 4
Oncology Case 4
Submitted by
Dr. Jasim Jaleel,
Assistant Professor,
Department of Nuclear Medicine,
Institute of Liver and Biliary Sciences, New Delhi, India.
Question
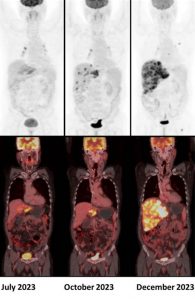
A 50 year old male, diagnosed with metastatic cholangiocarcinoma, was started on Durvalumab. Given below are his serial 18F-FDG PET/CT scans. Which of the following statement is correct ?
a) Scan findings are suggestive of stable disease (iSD)
b) Scan findings are suggestive of unconfirmed progressive disease (iUPD)
c) Scan findings are suggestive of confirmed progressive disease (iCPD)
d) Response assessment cannot be performed in this case.
Scroll down for answer
Answer: C. Scan findings are suggestive of confirmed progressive disease (iCPD).
Durvalumab is an immunotherapy drug in the class of programmed death-ligand 1 (PD-L1) inhibitors. Recently, several attempts to establish adapted imaging criteria for follow-up to immunotherapy treatment have been proposed, notably, the immune Response Evaluation Criteria in Solid Tumors (iRECIST) and Immune PET Response Criteria in Solid Tumors (iPERCIST) (1).
According to the iPERCIST, complete resolution of FDG uptake within the target lesion is considered a complete response. A more than 30% decrease in the target tumor FDG SULpeak is considered as partial response. A more than 30% increase in FDG SULpeak or the advent of new 18F-FDG-avid lesions is considered an unconfirmed progressive disease, which should be confirmed by a second follow-up scan 4-8 weeks later. If progression was observed in the second scan, it is then labelled as a confirmed progressive disease. If there is no significant interval change (less than 30% increase or decrease in SULpeak with no new lesions), it is labelled as stable disease. If there is more than 30% decrease in the target tumor FDG SULpeak, then it is considered as partial response (2).
Pseudoprogression occurs when the tumor burden initially increases due to an increase in lesion size and/or the occurrence of newly detectable tumor lesions with a subsequent decrease in tumor burden (3). Another atypical response pattern related to immunotherapy is hyperprogression, a term with various definitions that indicates a pronounced acceleration of tumor growth. If hyperprogression is suspected, treatment must be interrupted immediately, although robust biomarkers are still pending (4).
Various other PET-related response criteria in immunotherapy include PET/CT Criteria for Early Prediction of Response to Immune Checkpoint Inhibitor Therapy (PECRIT) and PET Response Evaluation Criteria for Immunotherapy (PERCIMT) (5).
References:
- Costa LB, Queiroz MA, Barbosa FG, Nunes RF, Zaniboni EC, Ruiz MM, et al. Reassessing Patterns of Response to Immunotherapy with PET: From Morphology to Metabolism. RadioGraphics. 2021 Jan;41(1):120–43.
- Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019 Jan 29;9(1):8.
- Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015 Nov;33(31):3541–3.
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017 Apr 13;23(8):1920–8.
- Unterrainer M, Ruzicka M, Fabritius MP, Mittlmeier LM, Winkelmann M, Rübenthaler J, etal. PET/CT imaging for tumour response assessment to immunotherapy: current status and future directions. Eur Radiol Exp. 2020 Nov 17;4(1):63.